 Available for Import
Available for Import
Coronary cobalt-chromium NA SH coronary stent on delivery system 2.75x39mm
Manufacturer:
Price:
Request Quote
Bulk pricing available
FOB, CIF & EXW terms available
Key Highlights
- Made in Russia - engineered for durability
- Suitable for challenging environments
- Lower cost of ownership than European alternatives
- Bulk orders available with volume discounts
- Documentation and customs clearance assistance provided
Available Models
Coronary cobalt-chromium NA SH coronary stent on delivery system 4.5x14mm
Coronary cobalt-chromium NA SH cobalt-chromium stent on delivery system 2.0x34 mm
Coronary cobalt-chromium NA SH cobalt-chromium stent on delivery system 2.75x24mm
Coronary cobalt-chromium NA SH cobalt-chromium stent on delivery system 3x24mm
Coronary cobalt-chromium NA SH coronary stent on delivery system 2.5x8mm
Description
Cobalt-chromium coronary stent NA SH on the delivery system, designed for implantation in the coronary artery to maintain cavity patency of the vessel and increase the diameter of the lumen.
The field of application is cardiovascular surgery.
The stent is used during angioplasty.
Indications for use:
In patients with ischaemic heart disease:
- "de novo" stenosis or restenosis of the coronary artery ø 2-4,5 mm;
- occlusion of cardiac bypass shunts ø 2-4.5 mm, including saphenous vein shunts.
In patients with acute myocardial infarction:
-obturation of the coronary artery ø 2-4.5 mm
The stent system consists of a delivery system with a balloon at its distal end and a stent placed on the delivery system over the balloon.
The stent is a permanent implant made of CoCr alloy L605. Stents are manufactured in various internal diameters (in mm): (2.00, 2.25, 2.50, 2.75, 3.00, 3.50, 4.00, 4.50) and lengths (in mm): (8, 9, 13, 14, 16, 19, 24, 24, 29, 32, 34, 37, 39, 40).
The field of application is cardiovascular surgery.
The stent is used during angioplasty.
Indications for use:
In patients with ischaemic heart disease:
- "de novo" stenosis or restenosis of the coronary artery ø 2-4,5 mm;
- occlusion of cardiac bypass shunts ø 2-4.5 mm, including saphenous vein shunts.
In patients with acute myocardial infarction:
-obturation of the coronary artery ø 2-4.5 mm
The stent system consists of a delivery system with a balloon at its distal end and a stent placed on the delivery system over the balloon.
The stent is a permanent implant made of CoCr alloy L605. Stents are manufactured in various internal diameters (in mm): (2.00, 2.25, 2.50, 2.75, 3.00, 3.50, 4.00, 4.50) and lengths (in mm): (8, 9, 13, 14, 16, 19, 24, 24, 29, 32, 34, 37, 39, 40).
Specifications
Stent length
39 mm
Nominal stent diameter
2.75 mm
Material
Cobalt-chromium alloy
Share your requirements for a quick response!
Instant response in 15 minutes
Best wholesale prices guaranteed
Direct from manufacturer
Delivery & Payment
Shipping Terms
Delivery Time
Sea freight: 30-60 days (depending on destination)
Air freight: 14-21 days (for urgent orders)
Payment Methods
Similar Products You May Be Interested In
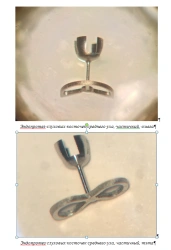
Endoprostheses of the auditory ossicles of the middle ear, ESC-"CONMET", partial according to TU 32.50.22-030-11458417-2021
View Details
Distraction cage, Ø16 mm diameter, article no. 841.556.
View Details
Full-flow heart valve prostheses "MEDINGE-ST" made of pyrocarbon with attachable cuff, with accessories according to TU 9444-015
View Details
Interbody oval lumbar cage, height 8-16 mm, convergence angle 8°, part number 836.8XX
View Details
Therapeutic and training tibial prostheses
View Details
Endoprostheses of auditory ossicles of the middle ear, ESC-"KONMET", total according to TU 32.50.22-030-11458417-2021.
View Details
Blood vessel prosthesis synthetic knitted linear covered with collagen D 10mm L 20cm
View Details
Toothed lid, Ø26 mm with tilt angle from 0° , item no. 841.300.
View Details
Polymer mesh endoprostheses for reconstructive surgery and guides for their installation, sterile according to TU 9393-007-56257
View Details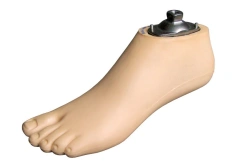
Mid-heeled foot 9A 018 type Sach female adult 25 PJSC RKK Energia Russia
View Details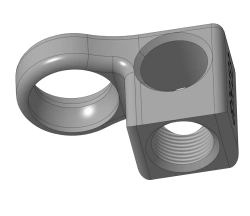
Cruciform block, article no. 839.133
View Details
Set of carbon implants for formation of musculoskeletal residual limb after enucleation of eyeball and other plastic surgery.
View DetailsVerified Suppliers
All products are sourced directly from authorized Russian manufacturers
Quality Assurance
Products meet international quality standards with proper certification
Global Shipping
Reliable logistics solutions to deliver products to your location
Secure Payments
Multiple secure payment options to facilitate international transactions